News Story
'Acid rain' Perhaps not so Detrimental to Outdoor Sculptures
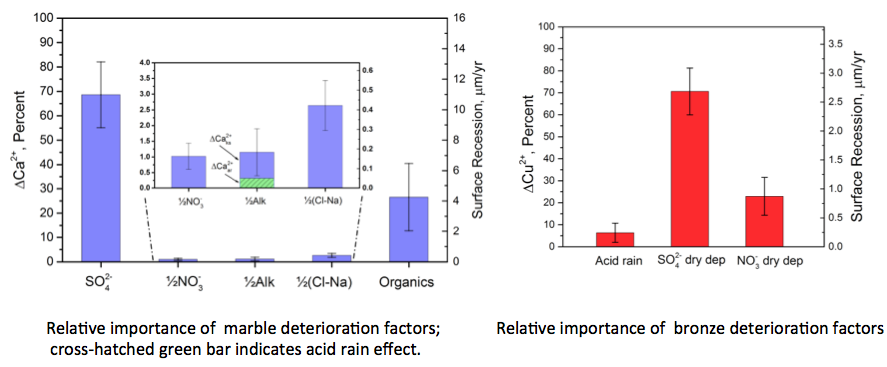
The preservation of cultural heritage for posterity is a core value of society around the globe. Works of art exposed to the outdoor environment are subject to attack by a variety of agents of degradation. Air pollution has been identified as a significant man-made cause of deterioration and specifically there is a widespread perception that acid rain is a major threat.
MSE Adjunct Professor Richard Livingston recently developed a geochemical modeling approach and applied it to two case studies to test the validity of this perception. Using the ‘mass balance’ method, where input = output + accumulation, Dr. Livingston analyzed the rainfall/runoff datasets from a marble statue located in New York City, and another on a trio of bronze (i.e. copper) memorial plaques in Gettysburg, Pennsylvania. In both cases, Livingston concluded that sulfur dioxide gas or acidic particles in the ambient air (i.e. dry deposition), is more to blame than acid rain for the degradation of these monuments. In the case of marble, man-made or natural organic acids may also be a significant factor.
Dr. Livingston’s paper entitled, “Acid rain attack on outdoor sculpture in perspective,” was published in the journal, Atmospheric Environment this month. To read the research in its entirety, please follow this link: http://www.sciencedirect.com/science/article/pii/S1352231016306227
Livingston has conducted a significant amount of research on materials degradation of cultural heritage including the Statue of Liberty, and is currently a Research Associate at the Smithsonian Institution’s Museum Conservation Institute.
Published December 14, 2016









