News Story
MSE Researchers Create Novel Liquid Crystal Materials From Particles
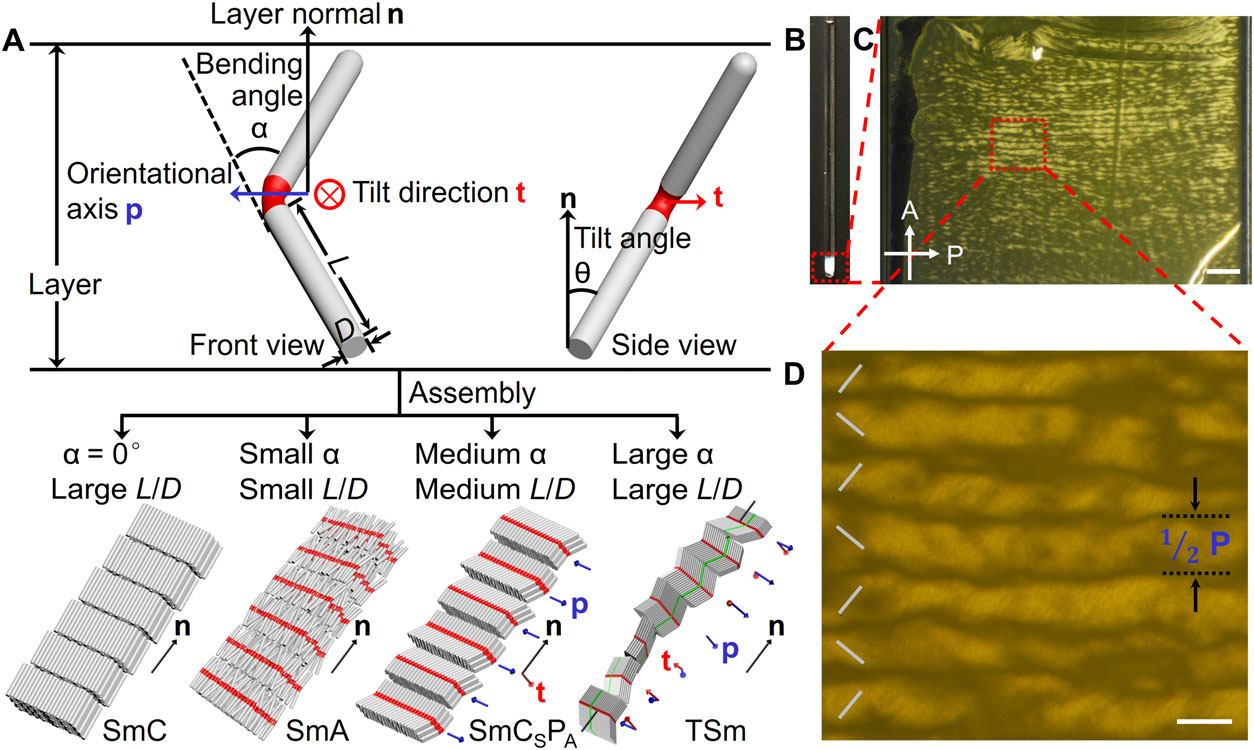
Schematic illustrations of the geometric and orientational parameters of BRs and the assembled SmC, SmA, SmCSPA, and TSm phases.
Researchers at the University of Maryland (UMD) have discovered a new type of liquid crystal (LC) based on particles. LCs permeate contemporary life - they're used in clock displays, calculators, smartphones, televisions and computer monitors, to name a few. Whereas LCs are organic materials, this study is based on inorganic materials that are abundant on the earth.
Scientists in the UMD Departments of Materials Science and Engineering (MSE) and Chemistry and Biochemistry (CHEM), have focused their efforts on exploiting the LC behaviors of particles that have the shapes of organic LC molecules, in order to develop cheaper and more efficient LC materials. This study was published online in Science Advances on May 11.
The three primary types of LCs - also called mesophases - are nematic, smectic and cholesteric. Cholesteric - often called the chiral nematic phase - changes color when exposed to various temperatures, which is why it's commonly used in thermometers, inks and paints.
The molecules of traditional LCs – being anisotropic – take the form of a disc or rod. The shape of the molecule, in part, affects the 'arrangement' that the LCs settle in. Straight, rod-shaped LCs – typically used in flat panel television displays (i.e., LCD TVs) – have been studied extensively for almost a century. The discovery of bent-core (also called banana-shaped) LCs has a much shorter history – roughly 20 years. They can exhibit rich new mesophases that are distinct from traditional straight, rod-shaped LCs and show promise in the use of electro-optical devices and light shutters.
What’s unique about this study is the use of bent rod-like colloidal particles to reproduce the LC behaviors of banana-shaped molecules. Compared with molecular LCs, colloid-based LCs are thermally stable, cheap and more sensitive to external fields, making them attractive in applications ranging from sensors to displays and metamaterials.
Imagine you toss a fairly large box of pencils onto a desk. Depending on the strength of the toss, the pencils will end up pointing in (roughly) the same direction. “Strength” represents an external field, such as temperature, or an electric or magnetic field.
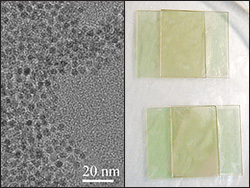
To make the ‘pencils’ easier to manipulate and align, "it's best to use colloidal materials, which have a gelatinous texture, for liquid crystals" said Yang Yang, a MSE/CHEM post-doc and first author on the study. “When the shape of rod-like particles is curved rather than straight, the rods align in a more complex manner, depending on their geometry," (e.g., bending angles, aspect ratios and bending position).
Colloids - like Jello, Mayonnaise or toothpaste - are somewhere between a solid and a liquid. They can be more stable than small molecules or polymers and not as temperature-sensitive, making them more efficient. There are situations when liquid crystals made of small organic molecules or polymers serve as scaffolding to give the colloids structure. The advantage of the colloids fabricated by this group is that they can adopt their own structures.
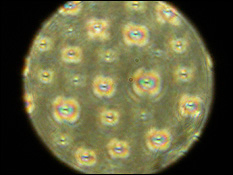
Dr. Yang's rods only take a few hours to grow are made of silica, which is basically beach sand – the most common mineral on Earth - meaning they're inexpensive to produce. When the rods slowly settled from a 30-day suspension in DMSO (dimethyl sulfoxide), they positioned themselves to form different ordered LC structures. The organization of rods was characterized by scanning electron microscope (SEM) and polarized optical microscopy (POM). A theoretical model was developed to confirm and predict the LC behaviors of bent rods.
"You can use electric fields to orient them, which offers variations in color and transparency," said Dr. Yang. Although these rods are achiral in nature, they can pack in a chiral manner to maximize a system’s efficiency.
"This can give rise to ferroelectric behavior, which requires a lower electric field to make the molecules change direction," said MSE professor and co-author on the study, Luz Martínez-Miranda.
"We can make large enough quantities to continue studies like this," said MSE professor Isabel Lloyd, another co-author. "In other techniques, you don’t have enough rods to even think about organizing them. This method could have dental applications, too. This is one step in the process of being able to mimic the microstructure of what your teeth look like under a microscope, in a polymer composite."
Lloyd added, "This is a fundamental study that will lead to bigger and better things, and even more applications.”
For additional information: Yang, Y., Pei, H., Chen, G., Webb, K., Martinez-Miranda, L., Lloyd, I., Lu, Z., Liu, K., Nie, Z. “Phase behaviors of colloidal analogs of bent-core liquid crystals.” Science Advances, 11 May 2018. DOI: 10.1126/sciadv.aas8829
Published May 11, 2018